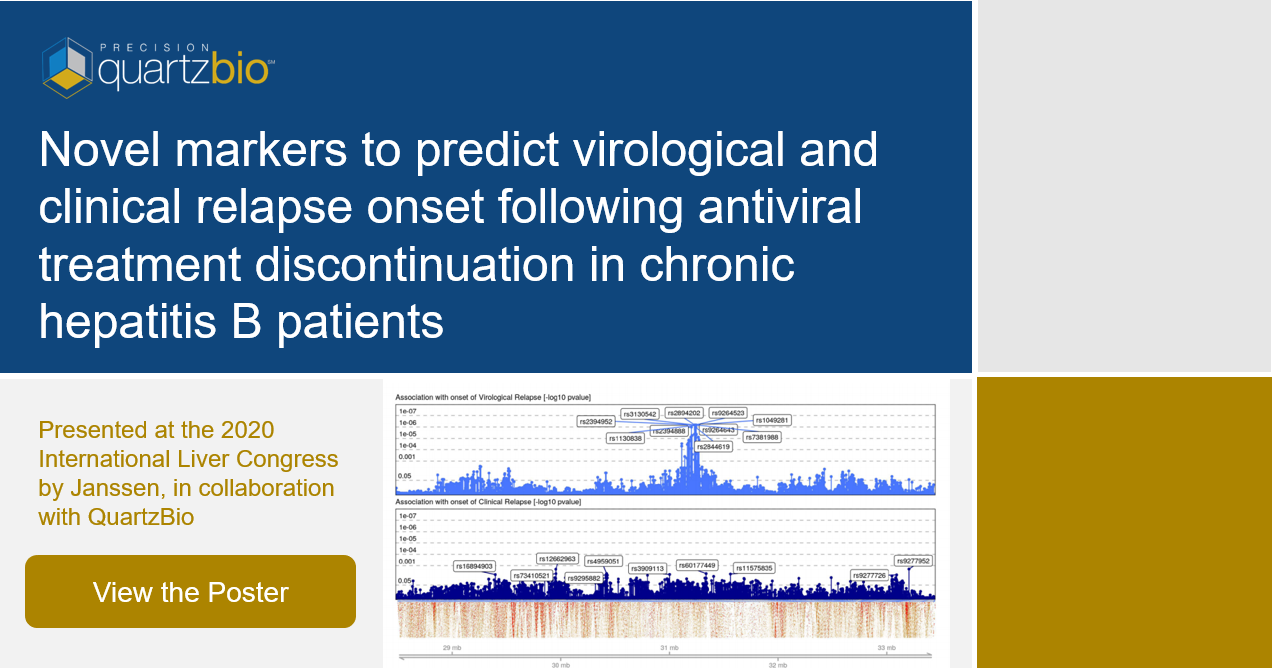
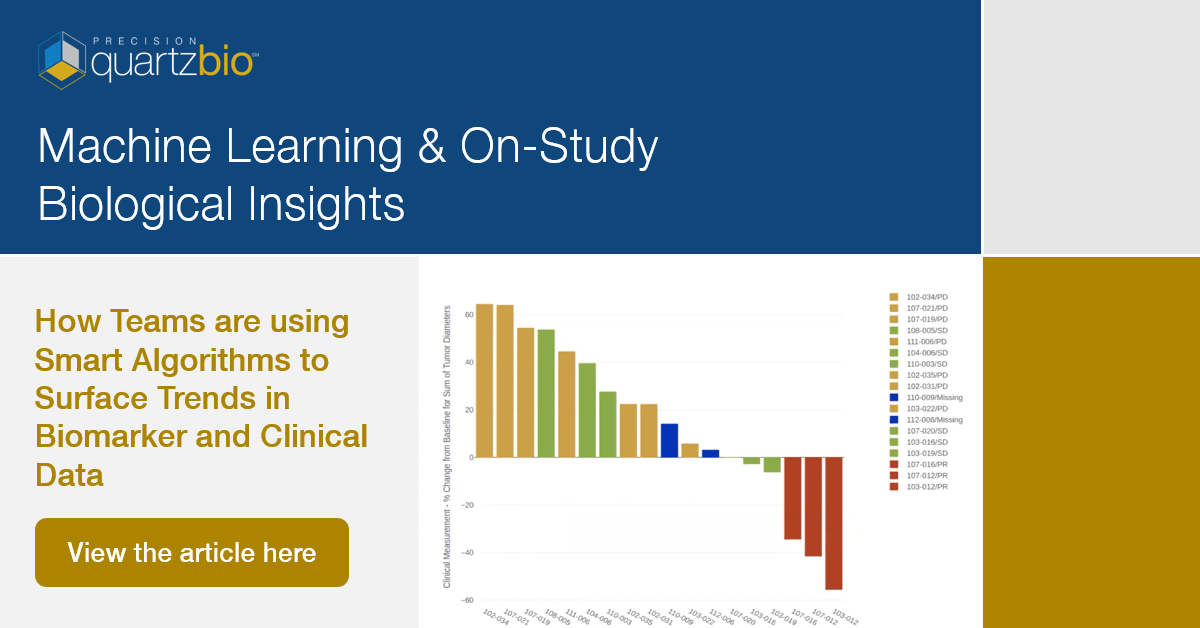
Title: Transcending Biomarker Data Chaos: How Integrating Disparate PK, Clinical & Exploratory Data Enables Deep Translational Insights
Duration: 30 minutes
You will see how teams unlock translational intelligence:
- Navigate vast exploratory data – identify trends and data points of interest
- Surface insights – analyze biomarker trends in collaborative dashboards across subject IDs, time points, response status, and treatment groups
- Access data rapidly – point and click navigation to underlying data files to interrogate the raw data behind the summary values
- Interrogate cross assay – map to a common data dictionary to enable rapid interrogation of trends across assay modalities: within and across studies