QuartzBio Helps Keep Oncology Trial on Track at a Clinical-Stage Biotech
A clinical-stage biopharmaceutical company has been developing novel therapeutics that selectively modulate gene expression to address unmet medical needs in cancer patients.
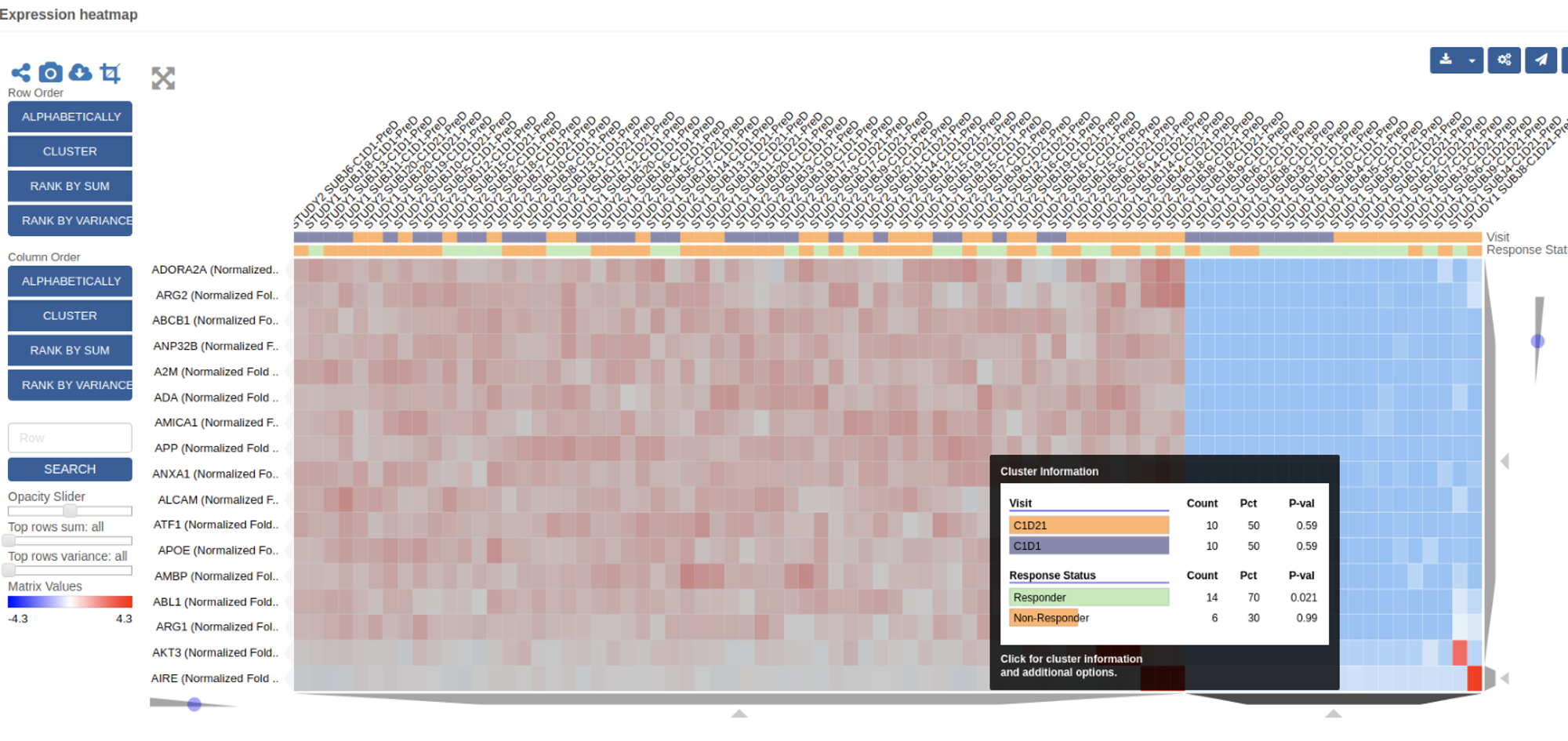
To keep their phase I study of an epigenetic modulator on track, the program’s translational research team needed to be able to monitor clinical outcomes with respect to patients and with respect to biomarker-defined subgroups.
To achieve this goal, the team needed to manage multiple data streams:
- Central lab sample inventory
- EDC/Clinical data
- Assay results data from multiple specialty labs, including IHC, flow cytometry, PK, and gene expression
Biomarker Data Management (BDM) & virtual Sample Inventory Management (vSIM)
In weeks, QuartzBio deployed its vSIM and BDM solutions to the clinical trial in progress, enabling the translational research team to:
- Gain centralized visibility into status of samples as they move from sites to central and specialty labs
- Revealed insights on actual samples collected vs. expected samples collected
- Generate hypotheses based on biomarker assay results linked to patient clinical data and dosage information
- Eliminate time-consuming and manual data cleaning and cross-referencing processes