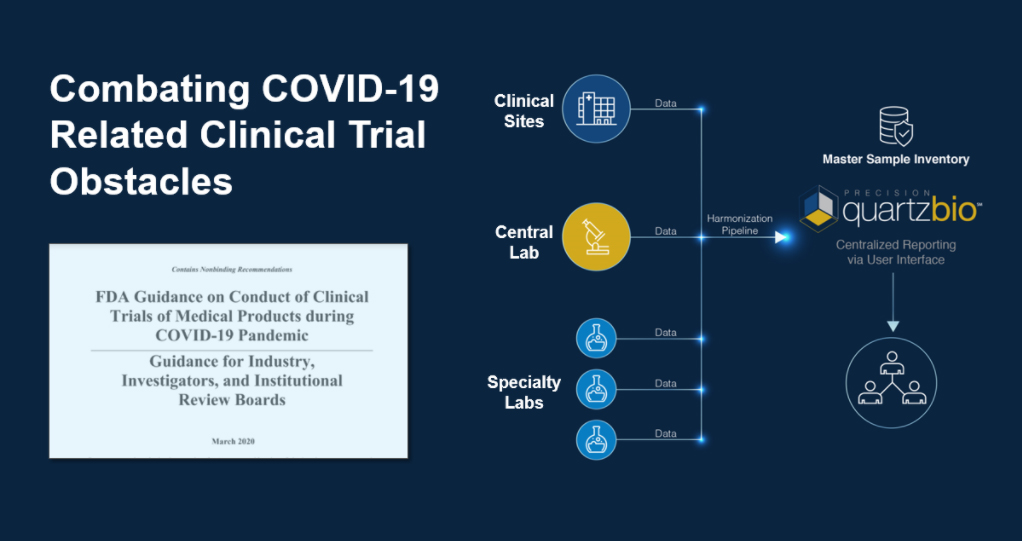
April 1, 2020 — The global pandemic caused by COVID-19 is presenting a public health crisis, and unprecedented challenges in the conduct of clinical trials. The quickly realized disruption has motivated FDA and EMA to issue guidance in direct response. As we continue to adapt to the new reality, we are seeing proactive monitoring of strained health care systems and potential for shipping disruptions as top of mind issues for Sponsors operating ongoing trials. This is especially true as Sponsors work to maintain R&D momentum and continue to serve patients – while adapting to an increasingly remote workforce requiring virtual collaboration.
Read more