by Tobi Guennel, PhD, SVP of Product Innovation, QuartzBio
I just read Microsoft’s latest Work Trend Index report, “The Year the Frontier Firm Is Born,” and it resonates deeply with the challenges and opportunities we’re seeing in precision medicine R&D.
The report highlights a seismic shift: the rise of AI agents isn’t just about automating tasks; it’s about fundamentally rewiring how businesses operate.
Key takeaways from the report include:
- Intelligence on Tap: AI is becoming an abundant, on-demand resource, offering “digital labor” to bridge the gap between increasing business demands and human capacity.
- Human-Agent Teams: The future isn’t human vs. machine, but human + machine. Teams will increasingly integrate AI agents as “digital colleagues,” shifting the focus from functional silos to outcome-driven “Work Charts”. This requires a new mindset, viewing AI not just as a tool, but a thought partner.
- Every Employee an Agent Boss: Managing and directing AI agents will become a core skill across all levels, accelerating careers and enabling more strategic work earlier.
This vision of AI-augmented work mirrors the complexities we navigate daily in the precision medicine value chain.
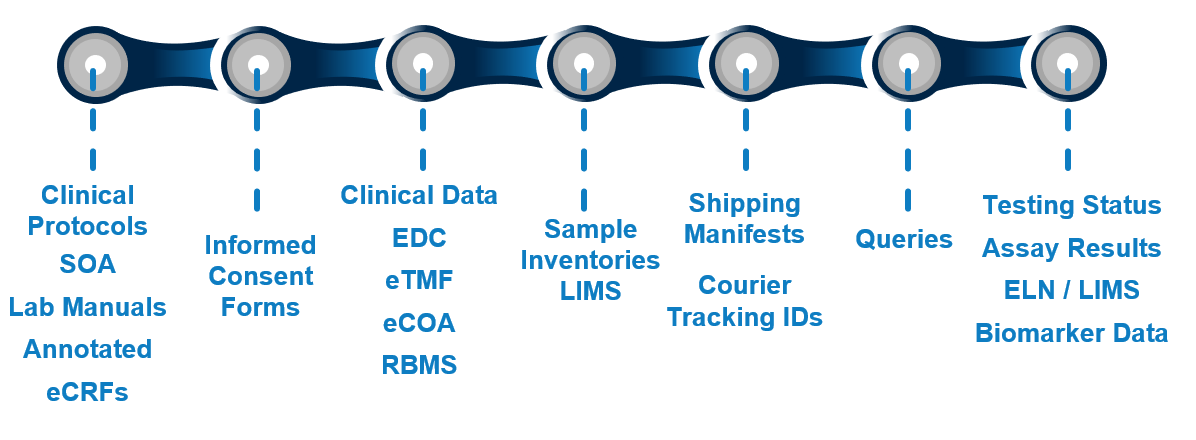
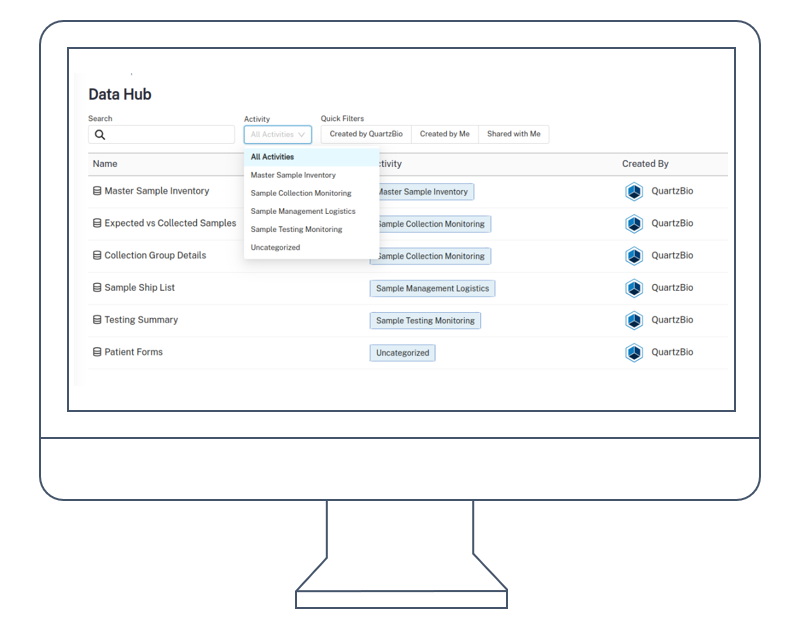
Currently, getting answers often involves a complex “information ping-pong” across various teams and systems, delaying critical insights. Clinical trials generate vast amounts of data, yet extracting timely value remains a significant hurdle for many organizations.
At QuartzBio, we believe the future lies in connecting this intricate value chain. We envision a future empowered by a seamless, semi-autonomous system – our Precision Medicine AI Agent Platform. This network of specialized AI agents is designed not to replace human expertise, but to amplify it.
Our platform aims to:
- Autonomously ingest and manage complex data streams across the R&D ecosystem.
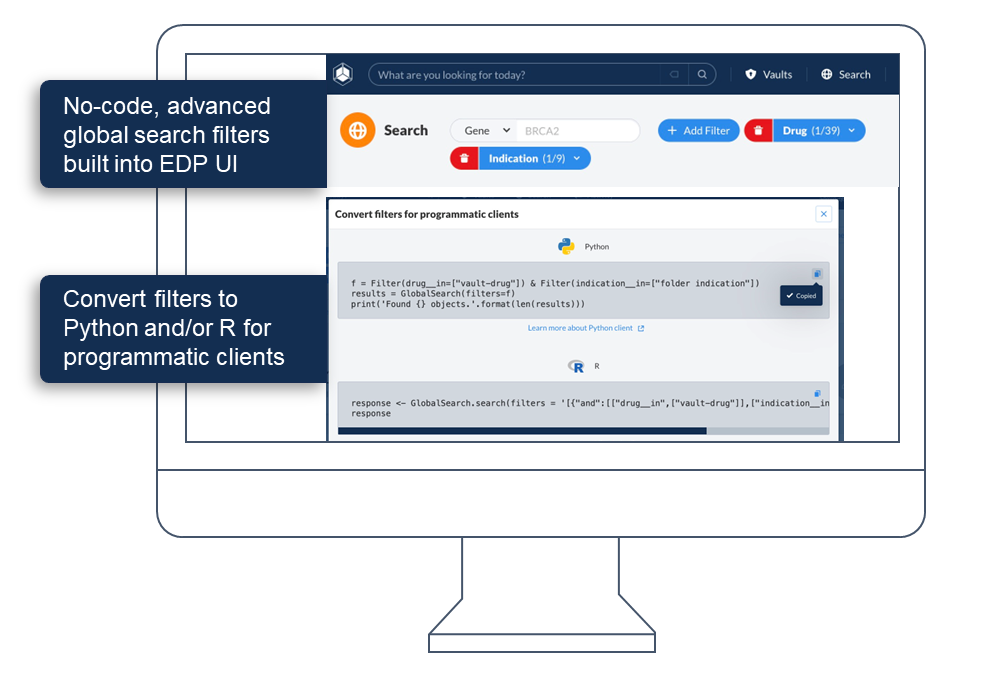
- Enable conversational interaction, allowing researchers and operational teams to interrogate data and receive insights in seconds, not weeks.
- Proactively deliver rich, contextual information to support strategic decision-making.
- Foster interoperability and collaboration, breaking down data silos.
By integrating human ingenuity with intelligent agents, we can shift the focus from manual processes to strategic insight generation, ultimately accelerating the delivery of novel treatments.
The “Frontier Firm” concept isn’t just a future hypothetical; it’s the operational model we are building towards in life sciences today.