The client: Biologics company developing cell therapies for blood cancers, generating large amounts of genomics data across four clinical programs and preclinical research teams
The client’s bioinformatics teams approached QuartzBio seeking a better workflow for sharing insights obtained from their genomics data.
They were using complex, manual workflows to ingest and process next-generation sequencing (NGS) data. These workflows comprised multiple steps, including alignment processing, variant processing, annotation, and clinical interpretation in the context of clinical data and publicly available data.
The challenge: Complex data management workflows hindered decision-making and created bottlenecks
The company had made a large financial investment in their data-rich therapeutic development programs – approximately $150M across the portfolio to date.
However, the complexity of their bioinformatics workflows made it difficult to share reports, analyses, and other visualizations with these stakeholders to get necessary signoffs and approvals.
Other specific concerns included
- Data integrity
- Data security and regulatory compliance
- User access management – not possible with existing workflows
The solution: Centralized visibility across a wide user base with QuartzBio® enterprise Biomarker Data Management (SaaS) solution
The client’s bioinformatics and translational medicine teams worked with QuartzBio’s genomics experts and data engineers to deploy the enterprise Biomarker Data Management (eBDM) solution.
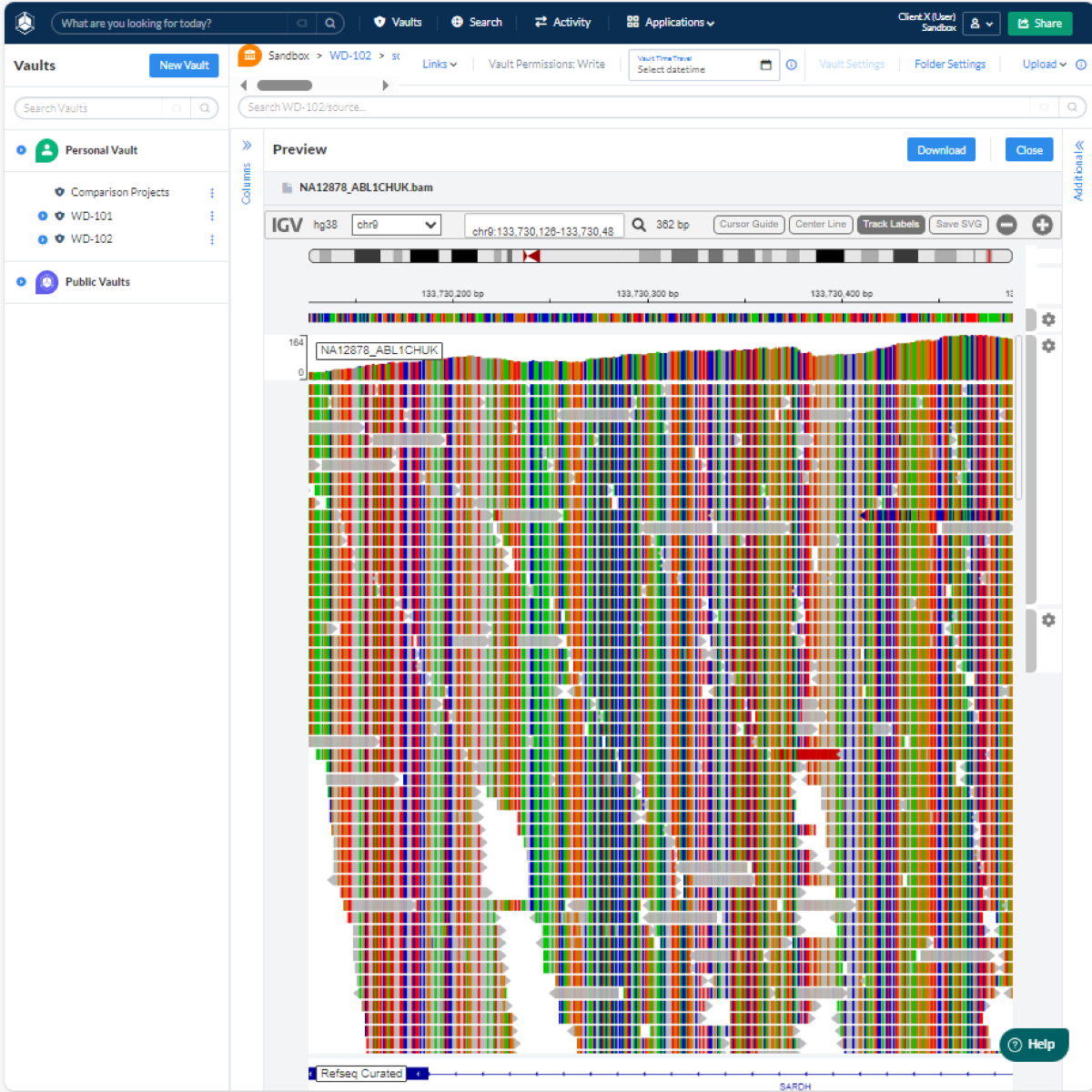
QuartzBio® eBDM, an enterprise SaaS solution, provided intuitive data query and topline reporting on data availability, enabling seamless data consumption across a wide user base. Bioinformatics teams were able to give stakeholders access to a centralized data vault where they could search, navigate, and explore all raw and processed data related to their genomics workflows (Figure 1).

To address the client’s concerns around data integrity, the eBDM solution included automated checks for data integrity and conformity with data transfer agreements (DTAs), providing transparent quality control of incoming data. All data workflow processes were set up in the eBDM solution with secure, 21 CFR part 11-compliant audit trails.
The outcome: Streamlined stakeholder approvals + integration with internal workflows = faster decisions
Deploying the QuartzBio® eBDM solution automated processes for cleaning and harmonizing the client’s genomics data and centralized it within a single solution.
The solution was further integrated with internal client workflows via API capabilities and native R/Python support, shortening the time from data acquisition to insight generation.
Most importantly, the bioinformatics teams could streamline stakeholder approvals by using a solution with both data security and fine-grained user access management.

