The client: Clinical development and translational research teams executing phase I and II studies for precision oncology
A clinical-stage biotechnology company developing precision oncology therapeutics recently worked with QuartzBio to tackle challenges facing execution of three phase I and two phase II biomarker-informed studies.
Challenges facing clinical development included:
- Limited pool of potential patients to recruit for studies
- Challenges facing patient screening and stratification – ideal biomarker profile not well-defined
Specific challenge: integrating siloed data
The clinical development team was challenged to solve the problems of patient qualification and stratification by integrating data from multiple streams, across multiple clinical programs:
- Electronic medical records
- Lab data
- Biomarker profiling with indication data for patients
- Immune profiling data
- Publicly available data
- Preclinical data: cell data and animal (cytokine and biomarker) data
The data sets being generated as a result of the clients’ clinical programs were scattered across multiple, disconnected cloud storage solutions. The team needed data to be integrated in a unified, query-friendly database that complied with regulatory requirements.
The solution: QuartzBio® enterprise Biomarker Data Management (eBDM) Software-as-a-Service (SaaS) Application
The client’s clinical development team worked with QuartzBio’s informatics subject matter experts and data engineers to deploy the eBDM platform within 6 weeks.
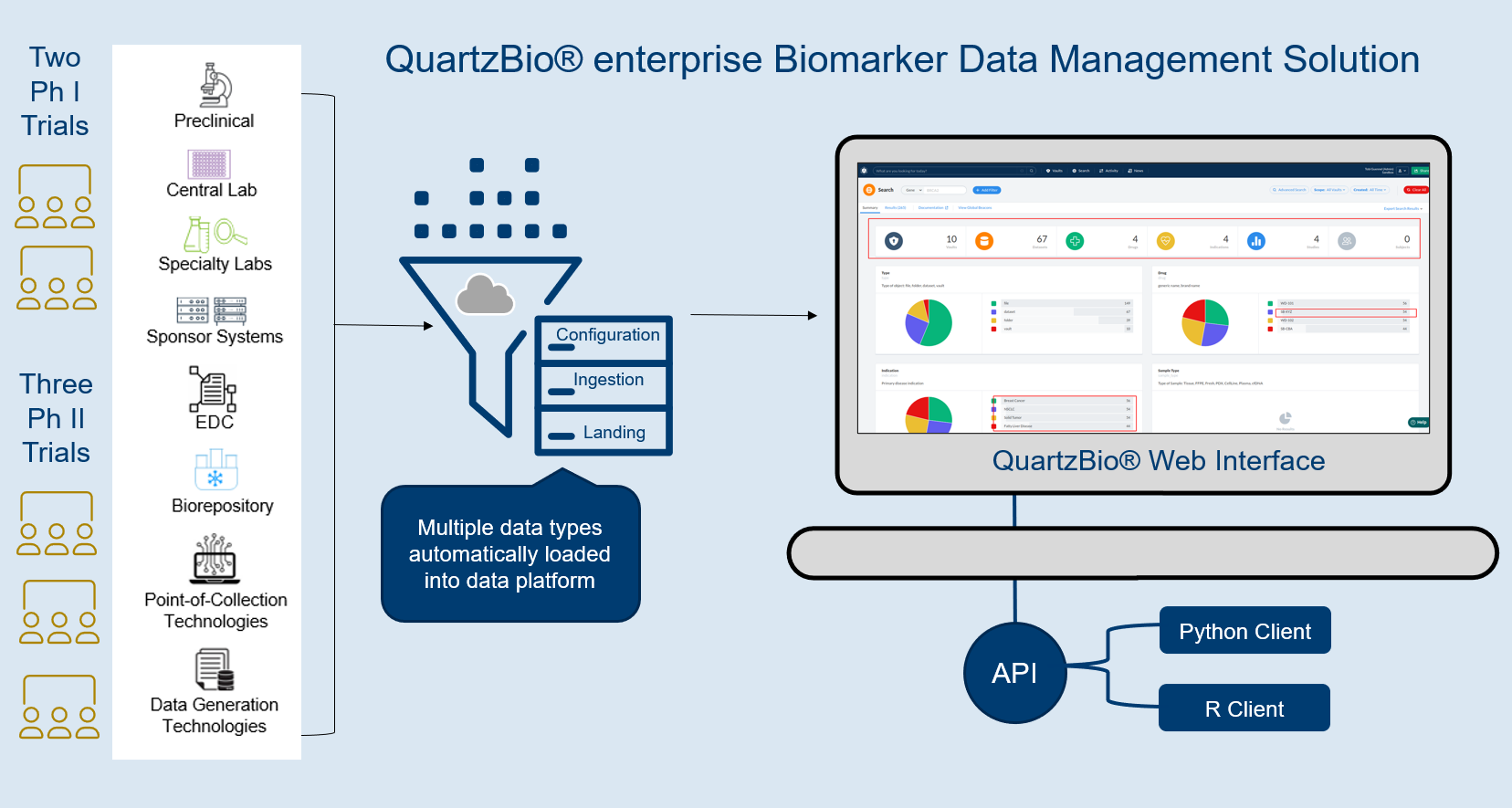
QuartzBio® eBDM, an enterprise SaaS solution, enabled scalable, user-driven data management of the client’s entire ecosystem of biological data (Figure 1).

Because the QuartzBio® data platform is data type-, data source-, and vendor-agnostic, every one of the clients’ data streams could be acquired, ingested, quality-controlled, and harmonized within the platform.
- Data type-specific templates were used to automatically harmonize and annotate data from different vendors at the time of or after data import
- QuartzBio’s library of configuration-ready workflows spanned all assay technologies used by the client: genomics, high-content imaging, flow cytometry, immunohistochemistry
The outcome: freedom to explore and transform data via web-based UI
Once data was set up within the platform, users had the freedom to explore and transform data via a web-based user interface (UI) with data access controls, version history and audit trails.
The enterprise Biomarker Data Management application enabled collaboration among a diverse user base across the client’s organization. Not only did the clinical development team uncover insights to inform trial execution, but the clients’ bioinformatics, translational biology, and biomarker operations teams also used QuartzBio’s visualization, data analysis, and top-line reporting capabilities as follows:
| Functional Team | How the team used enterprise Biomarker Data Management |
| Biomarker Operations | On-study reporting of data quality, data conformity, data availability, vendor performance, and turnaround times. |
| Bioinformatics / Computational Biology | Generated visualizations (e.g., line plots, clustering, dimensionality reduction) and were able to connect data to existing tools (e.g., SpotFire, Prism) |
| Translational Research | Obtained early insights into the ideal genomic profile of patients that showed response to the therapeutic candidate.  |

