Title: Overcoming Clinical Sample Chaos: A Critical Step in Biomarker-Rich Trials
Duration: 45 minutes
You’ll see how a technology-enabled approach to clinical sample inventory management delivers:
- Sample KPI dashboards for clinical, translational, & data management teams
- Visibility into sample collection, processing, and consent status
- Dynamic visualizations to quickly review expected vs actual sample status by patient, time point, and assay type
Abstract of the Webinar
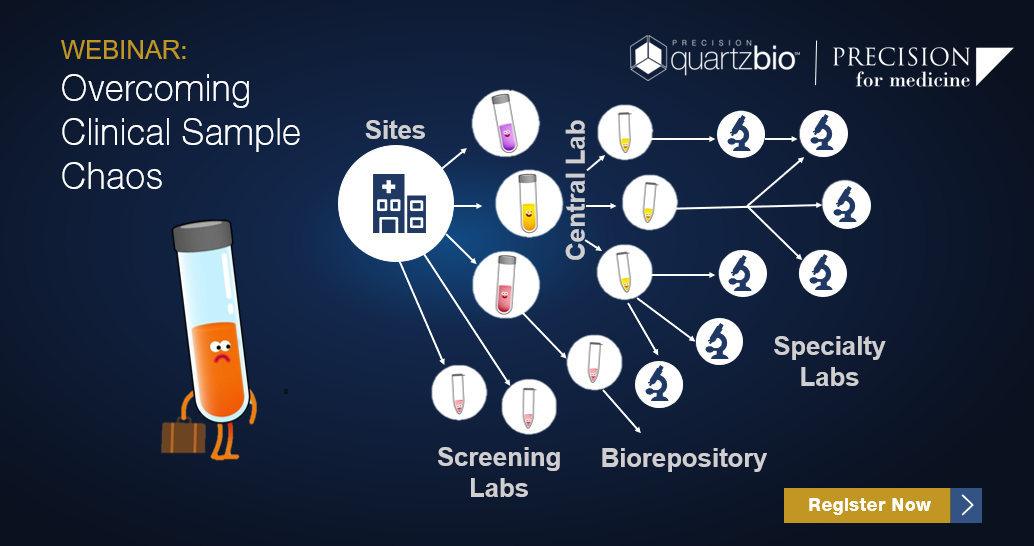
Biomarker data generation involves a complex network of sites, central labs, biorepositories, and specialty testing labs. With multiple sample types, derivatives, and time-points – keeping track of samples and data generation timelines presents a significant challenge.
Maintaining up-to-date sample records all too often requires manually searching inventories from multiple vendors and source systems (LIMs, PDFs, and Excel) – and then comparing against the protocol to make sure things are going according to plan.
Join us to see how clinical operations and translational teams are creating “Sponsor-centric” visibility into sample status across the lifecycle of collections, processing, testing, and storage.
About the Presenter:Tobi Guennel, Ph.D. |
QuartzBio, part of Precision for Medicine, is providing a technology-enabled next-generation data science solution to accelerate drug development. As the Chief Architect of our Data Integration and Informatics Platform, Tobi Guennel supports life science companies in their quest to improve patient care and outcomes. |

